Two health foods containing co-enzyme Q10, one containing melatonin, and one containing the fungus Ganoderma lucidum were approved via the filing track between March and June 30, based on a report by China regulatory firm CIRS.
Health foods containing co-enzyme Q10, melatonin, Ganoderma lucidum, spirulina, and fish oil were allowed to go through the filing track from March 1 – as they were added into the Health Foods Raw Materials Directory.
However, this regulation is only applicable to local Chinese firms. Overseas firms exporting such products into China for the first time will still need to go through the registration route – a more stringent and time-consuming procedure.
One of the co-enzyme Q10 approvals and the only approval for melatonin have gone to Shaanxi Shenlong Pharma, while the remaining co-enzyme Q10 approval was given to Sanyuan Huazhou Pharma and Ganoderma lucidum to Shaanxi Da Zhi Pharma.
The report also highlighted three health foods made in new formats were approved via filing.
They include two gummies containing calcium produced by Sirio Pharma and one powder product containing Ganoderma lucidum made by Shaanxi Da Zhi. Both are China-based OEM companies.
Companies are allowed to go through the filing track for gummy and powder health foods since June 1. Previously, health foods filing was limited to only tablets, hard capsules, soft capsules, oral liquid, and granules.
“The first half of 2021 had seen quick market entry of gummies and powder via the filing track. The implementation of these regulations has allowed companies to have a greater room to select the ingredients, function claims, and dosage formats when going for health foods filing,” the report said.
According to the report, a total of 614 health foods were approved via the filing track between January and June 30.
What’s common?
Multivitamins and minerals, vitamin C, as well as the combination of vitamin D and calcium, were the top three most approved health foods, with each given 122, 96, and 40 approvals respectively.
As for dosage formats, most products approved came in tablets (154) and soft gel capsules (150).
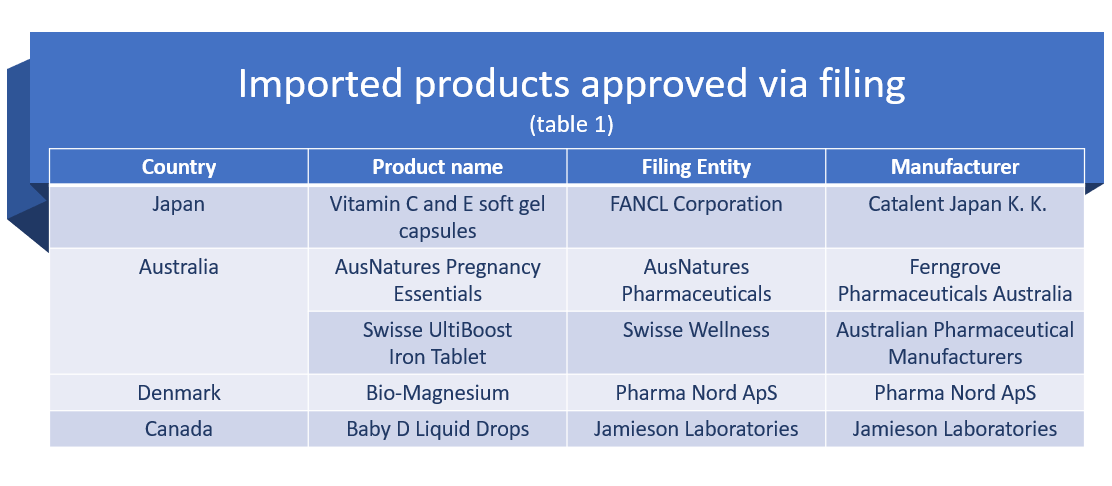
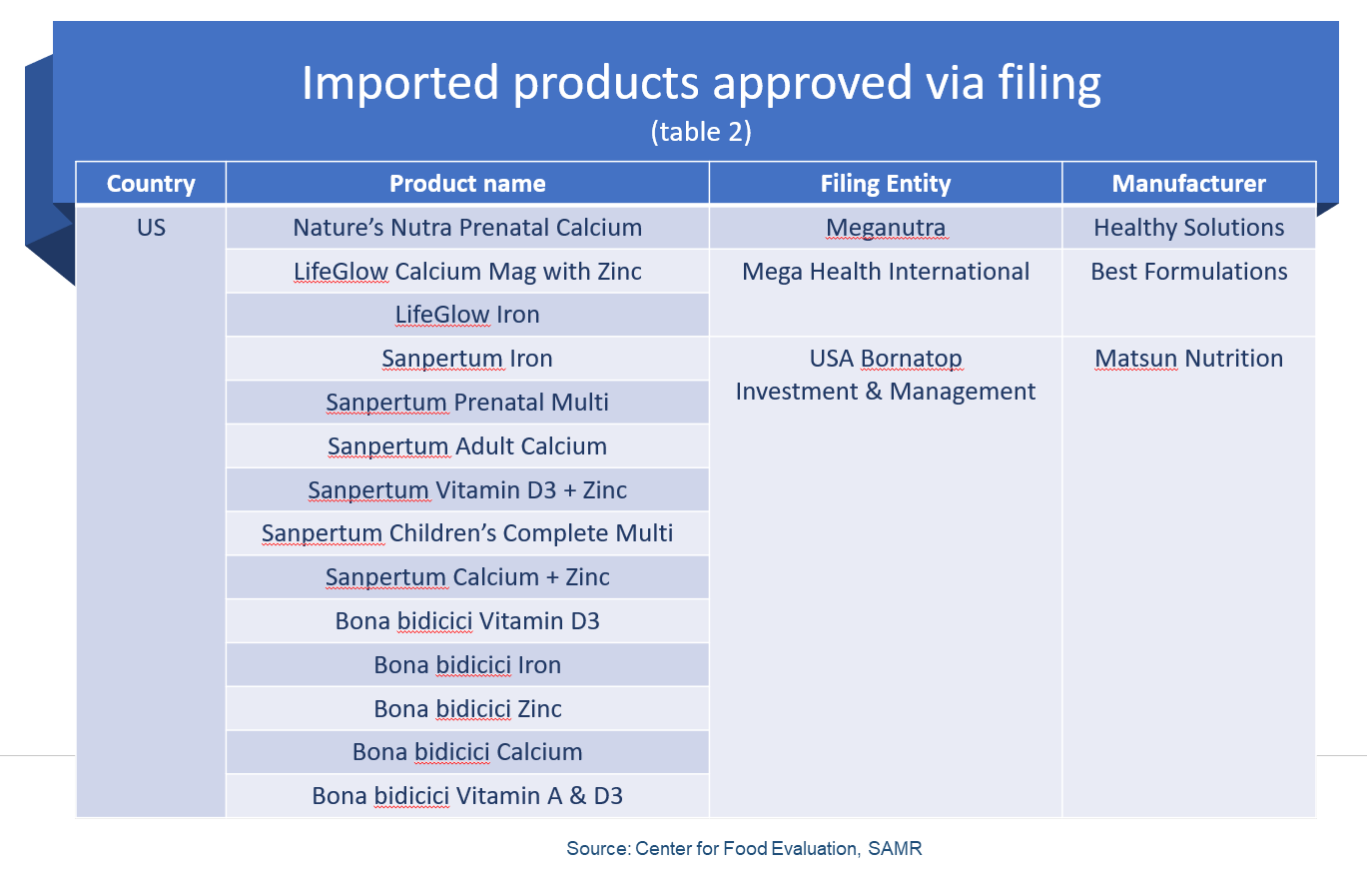
Imported products
Nineteen out of 614 approved health foods were imported products.
Of which, US dominated the charts with 14 health foods approval, followed by Australia with two approvals, and one approval each from Japan, Canada, and Denmark.
Most were oral liquid products (12), with five as tablets, one as soft capsule and one as liquid drops.