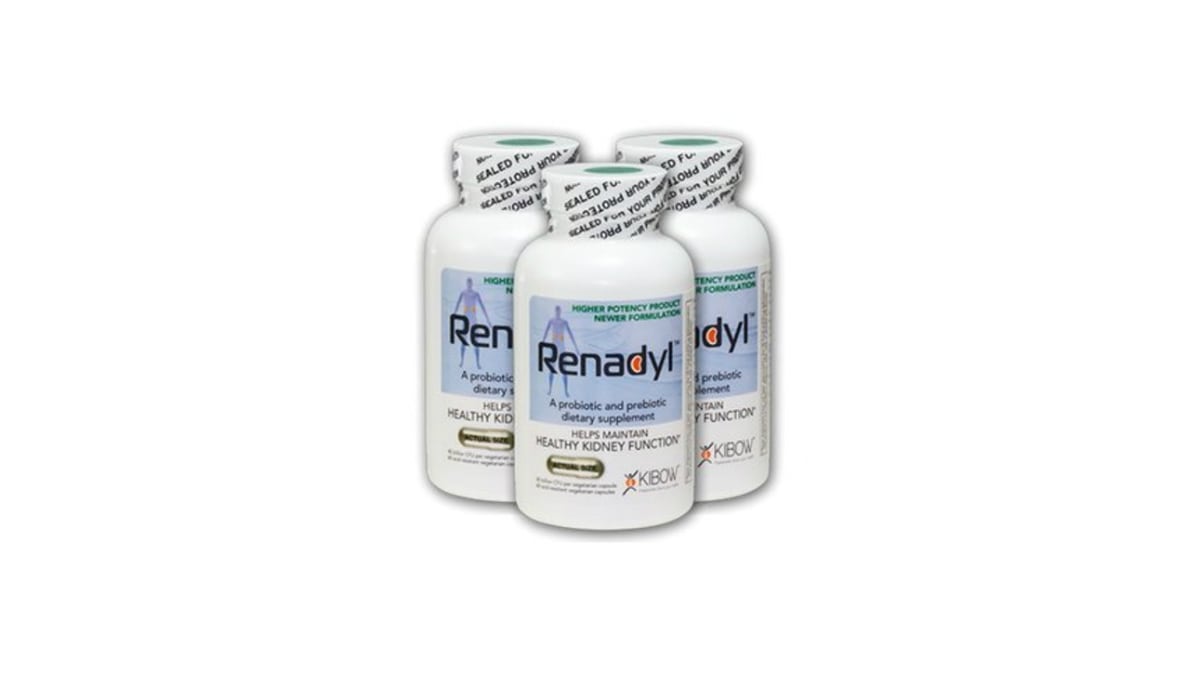
The proprietary product in question, Rendayl, is a prebiotic / probiotic supplement developed using Kibow's novel uremic toxin removal technology, which address the diffusion of uremic toxins into the bowel as a result of kidney failure.
Renadyl, which contains a combination of three specific probiotic microbial strains and prebiotics, is used by patients with moderate to severe kidney failure.
It is said to remove several uremic toxins — including urea, uric acid, creatinine, phenol, trimethylamine oxides, and cardiovascular toxins such as indoles — some of which are protein-bound and not removed by dialysis.
Life after lawsuits
In November 2017, Kibow initiated a collaboration with Centaur Pharmaceuticals in Mumbai, giving the latter exclusive rights to the import, marketing, distribution and sale of Renadyl in India; this was after Kibow's India patent had been granted in September 2008.
However, this was set against the backdrop of a longstanding patent dispute.
In a lawsuit filed in 2013 by Ahmedabad-based pharmaceutical firm La Renon Healthcare, the company challenged the validity of Kibow's patent before India's Intellectual Property Appellate Board (IPAB).
The IPAB eventually dismissed the challenge, upholding Kibow's patent. Now, the Chennai High Court has validated the IPAB order, effectively maintaining the patent again.
MD Natarajan Ranganathan told NutraIngredients-Asia: "La Renon filed a lawsuit with the IPAB, but that suit was dismissed and our patent upheld. However, even though IPAB had the final say, La Renon went ahead to file the lawsuit at the Chennai High Court, who again upheld our patent in January 2019."
So far, Kibow has obtained the same patent along with several others, and currently holds a total of 35 patents in Australia, Canada, China, Japan, Korea, the EU and the US.
The company is eyeing global growth, though Ranganathan said it will try to accomplish this "one country at a time", due in part to the often complex process of obtaining regulatory approval in different regions and countries.
Competitor confidence
Kibow has also entered the Kidney Innovation Accelerator (KidneyX), a public-private partnership between the US Department of Health and Human Services and the American Society of Nephrology to accelerate innovation in the prevention, diagnosis and treatment of kidney diseases worldwide.
Kibow is hoping its project proposal, entitled Enteric Dialysis, will win funding to attract greater investment and collaboration interest to the firm in the area of gut microbiome modulation within those with renal failure.
The company also hopes to obtain Live Bio-Therapeutic (LBT) drug classification for Renadyl, so it can qualify for medical reimbursement from federal government sources and private insurance firms.
At the same time, the firm recently formulated a new patented pre- and probiotic product for gout / hyperuricemia, for which it also intends to obtain LBT classification.
Underscoring the company's confidence in its formulae and products, Ranganathan said: "As of now, neither Kibow nor the US FDA has received reports of any adverse events from our novel uremic toxin removal technology.
"The only side effects may be minor symptoms like bloating, gas or flatulence, which may last for a week or two. However, these symptoms are reported by few patients and are self-corrected with the continuous use of our product.
"In fact, these initial side effects are a demonstration that our highly specific probiotic strains are competing with over 100 trillion microbes to consume uremic toxins."