Promotional Features
Dietary supplements: Building trust by ensuring quality
There is a global interest in diet supplementation—more than 50% of adults and children use dietary supplements with the motivation to improve or maintain overall health, and for site-specific conditions like heart, bone and joint, and eye health (l).
The passage of the Dietary Supplement Health and Education Act (DSHEA) in 1994 created a regulatory framework in the United States that allows the marketing of vitamins, minerals, herbs or other botanicals, amino acids, and their metabolites as dietary supplements, a new category of foods. During the last 26 years, the proliferation of new products has provided consumers access to about 85,000 dietary supplements in the US (2). Parallel or similar regulations are being considered around the world.
However, the presence of lower quality products in the market has raised concerns among consumers, health care practitioners, and manufacturers. For example, products may have included too little of the dietary ingredient, contain extraneous material, or may be adulterated with drugs or drug analogs (3-4). Ingredients in the supply chain may be misidentified, or contaminated with toxic materials.
In order to address the potential harm introduced by low quality ingredients and products, additional mechanisms to support the current regulatory requirements need to be in place to ensure the delivery of quality products to consumers. Industry self-regulatory practices, the use of public standards, verification services, and reference materials could be part of the preventive network to ensure quality.
Industry adoption of stringent self-regulatory actions may also help to alleviate the potential for negative public perception of the industry as a whole due to negative health impact derived from adulterated products. At the core of the supplement safety is the assurance of quality. Although a long history of safe use of a natural product may provide some assurance that the product may be used safely, this does not address safety concerns due to contamination with microbes, pesticides, or naturally-occurring elements, such as arsenic, lead, cadmium, and mercury that could occur through growing, harvesting, processing, or packaging conditions.
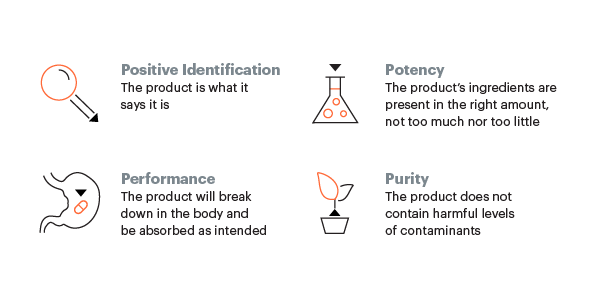
For high quality dietary supplements, identity and purity of the ingredients should be demonstrated, processed into finished products according to Good Manufacturing Practices, and accurately labeled.
Manufacturing challenges
Dietary ingredients are subject to multiple processes in manufacturing of the dietary supplements. For example, a botanical supplement may contain a raw material processed in different ways: it may consist of the plant material milled to a powder, a dry extract prepared with sol vents, or the isolated active constituents of the plant material. To ensure quality of these processed ingredients, it is important to use unambiguous names for the ingredients, as well as appropriate and scientifically valid test methods to establish identity and strength. For example, a test that relies on long intact DNA fragments may be appropriate species identification in a botanical raw material, but not fit for the purpose to establish identity of an ingredient prepared as a botanical extract processed with steps that degrade the DNA, or purified to leave out the nucleic acids. The current Good Manufacturing Practices (cGMPs) in the US for dietary supplements (5) were introduced in 2007 following Congressional mandate as established by the legislation in 1994. GMPs are a critical piece of the overall network to ensure quality. According to the rule, manufacturers are responsible for establishing proprietary and thus private specifications for the products they produce and the ingredients they use.
The recent incidents of economically motivated adulteration, such as amaranth dye in products marketed as bilberry extract, highlight the need for public specifications that are based on scientifically valid analytical methods (3). While the cGMPs mandate manufacturers to establish specifications, by not making these specifications publicly available, the determination of whether those specifications are met is sole responsibility of the manufacturer and consumers cannot confirm if that is actually the case. The lack of product quality specifications, and inadequacy of ingredient identification tests continue to be among the most common cGMP violations. The negative public health impact from these quality issues may be addressed through transparent quality standards (6).
Safeguarding the supply chain
As the globalization of manufacturing and distribution continues, the need for stronger systems to assess and ensure quality in dietary supplements becomes more important. Many dietary supplements and ingredients are sourced from countries around the world. Missing or inaccurate information about the dietary ingredient can have serious health consequences. For example, Chinese star anise (either as a food ingredient, supplement, or medicinal product) is prized for its reputed health benefits, while Japanese star anise is toxic and can kill a consumer if passed off as the former. Opportunities for adulteration by unscrupulous manufacturers occur at several stages along the complex supply chain, which can endanger safety of the products. New detection technologies, such as chromatographic, spectroscopic, and genomic testing, are helpful in detecting and preventing these dangers (7). Dietary ingredients and supplements are increasingly sourced in international trade. For example, most of the ascorbic acid (vitamin C) and chamomile consumed in US are sourced from other countries. The global dietary supplement market involves challenges with the supply chain integrity in ensuring product quality. The incidents involving harm from products marketed as dietary supplements that are labeled to contain dietary ingredients, but instead contain drugs or drug analogs, pose serious risk to public health.
Build trust in your supplements with USP
USP offers tools to help manufacturers and suppliers safeguard the dietary supplement supply chain in the global marketplace by combating adulteration of raw materials and finished products thus helping to ensure that what is on the label is in the bottle.
USP standards
To assist industry in the development and testing of dietary supplements, USP offers documentary standards (written monographs that describe specifications and tests for identity, strength, quality, and purity) (9) and Reference Standards (8) (highly characterized substances intended for use in conducting the quality control tests and analytical procedures associated with documentary standards). Use of USP standards can help limit the introduction of potential adulterants and contaminants into the supply chain and serve as a widely acknowledged quality benchmark in the buying and selling of dietary supplement products and their ingredients. The United States Pharmacopeia--National Formulary (USP-NF) is an official compendium of the US for drugs and dietary supplements. USP's compendial quality standards are developed and approved by leading independent scientific expert volunteers from industry, academia, and international governments, with participation from representatives of the US FDA. The USP Catalog features more than 3,600 items, including more than 800 dietary supplement-related monographs and approximately 300 Reference Standards for dietary supplements (e.g., amino acids, botanicals, vitamins and minerals, and fish oils).
Independent verification services
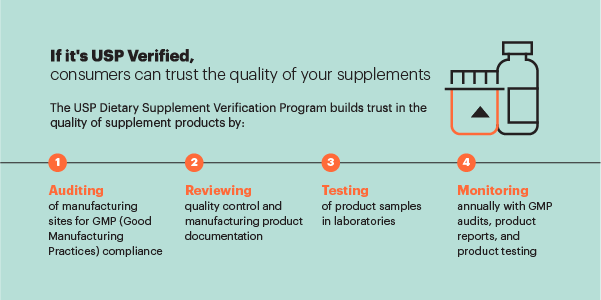
In addition to its standards-setting work, USP offers the industry voluntary, independent, third-party verification services for dietary supplement finished products and dietary ingredients. The USP Verified Mark is earned by dietary supplement products that meet the stringent criteria of its Dietary Supplement Verification Program. The Mark has appeared on more than 750 million supplement labels since the program's start in 2002—helping ensure that what's on the label is in the bottle in the right purity and strength. USP also has a GMP Facility Audit Program for dietary supplement and dietary ingredient manufacturers. The program helps ensure that manufacturers have good quality systems and may also help mitigate regulatory risks by preparing manufacturers for GMP inspection.
Meetings, courses, workshops, and roundtables
USP meetings, courses, workshops, and roundtables bring together the world's leading scientific, regulatory, and healthcare experts to share knowledge, lead discussions, and provide insight for the effective development and application of standards. USP's offerings have featured workshops on adulteration and DNA-based methods, making available the expertise of authorities drawn from government and other sources; and Expert Panels and roundtable meetings on challenging topics such as adulteration, probiotics, proteins, cranberry, pesticide residues, and DNA-based methods.
Set yourself apart
The competitive pressure and increasing dependence on a global supply chain to obtain raw ingredients are amongst the important factors contributing to a potential risk for the dietary supplement quality. The basis of contractual agreements between the sellers and buyers - the Certificate of Analysis - is important to help ensure the quality of final products. Using USP public standards and independent verification services helps ensure that quality creates certainty for consumers and regulators, advances transparency and fairness within the industry, and also helps keep dietary supplements that are tainted with drugs and drug analogs off the market.
References
- Bailey, R. L.; Gahche, J. J.; Miller, P. E.; Thomas , P. R.; Dwyer, J. T., Why US adults use dietary supplements. JAMA internal medicine 2013, 173 (5), 355-61.
- Dwyer, J. T.; Coates, P. M.; Smith, M. J., Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10 (l).
- Sarma, N.; Giancaspro, G.; Venema, J., Dietary sypplements quality analysis tools from the United States Pharmacopeia. Drug Test Anal 2016, 8 (3-4), 418-23.
- Pawar, R. S.; Grundel, E., Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs). Drug Test Anal 2017, 9 (3), 500-517.
- FDA. Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements; Final Rule. Title 21 Code of Federal Regulations Part 111, 111.1-111.610 (Revised April 1, 2011). 2007. http://edocket.access.gpo.gov/2007/07-3039.htm (accessed November 18, 2011).
- Miller, R.K.; Celestino, C.; Giancaspro, G. I.; Williams, R. L., FDA’s dietary supplement CGMPs: standards without standardization. Food and drug law journal 2008, 64 (4), 929-42.
- Simmler, C.; Graham, J. G.; Chen, S. N .; Pauli, G.F., Integrated analytical assets aid botanical authenticity and adulteration management. Fitoterapia 2017.
- USP USP Global Public Policy Position. Ensuring the Quality of Dietary Supplements. https://www.usp.org/sites/default/files/usp /document/about/public-policy/public-policy-dietary-supplements.pdf (accessed April 15, 2018).
- Schiff, P. L., Jr.; Srinivasan, V. S.; Giancaspro, G. I.; Roll, D. B.; Salguero, J.; Sharai, M. H., The development of USP botanical dietary supplement monographs, 1995-2005. Journal of natural products 2006, 69 (3), 464-72.